Treatment
Bob Martin Clear 2 in 1 Wormer Tablets for Cats & Kittens – Pack of 2
£5.99
- **PLEASE NOTE: Packaging may vary**
- An effective, one-dose treatment against the most common types of worms, roundworm and tapeworm
- Specially developed to make worming easy
- Suitable for cats and kittens over 12 weeks of age
- Clinically proven
Description
Bob Martin Clear Wormer 20/230 mg Tablets for Cats & Kittens
Bob Martin Clear Wormer Tablets for Cats and Kittens is a fast and effective treatment against all common types of intestinal worm that can normally infect cats in the UK. Most infected cats do not show signs of having worms; however, heavy burdens of worms can cause weight loss, vomiting and diarrhoea and failure to thrive.
Clinically proven, the tablets help to keep your cat healthy and worm-free when used routinely.
Effective from the first dose in eradicating tapeworms and roundworms.
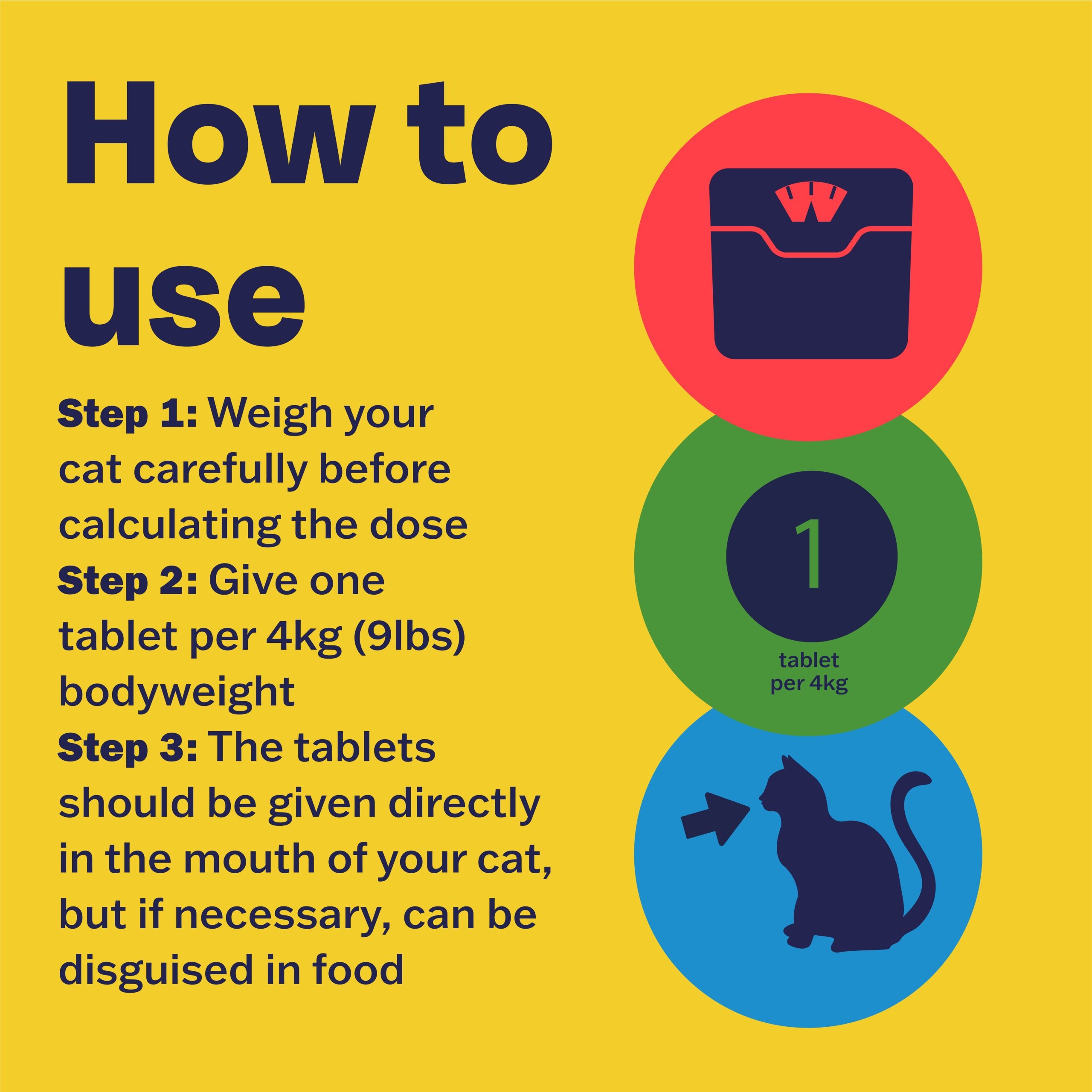
How to Use
Directions:
For animal treatment only.
Weigh your cat carefully before calculating the dose.
Give 1 tablet per 4 kg (9 lbs) bodyweight.
The coated tablets should be given directly but if necessary, can be disguised in food.
The tablets are scored into halves to enable a range of weights to be treated.
Weight of your cat:
1-2 kg: ½ tablet
Greater than 2 kg up to 4 kg: 1 tablet
Greater than 4 kg up to 6 kg: 1 ½ tablets
Greater than 6 kg: 2 tablets
For best results:
There is no permanent treatment for worms.
Veterinarians recommend that weaned kittens over 12 weeks old and adult cats should be wormed every 3 months as a routine measure.
It is also important to remove sources of possible re-infection such as fleas and mice.
If more frequent treatment is required and for advice on worming kittens less than 12 weeks old, consult a veterinary surgeon or a suitably qualified person
Active Ingredients
Each tablet contains 20 mg praziquantel and 230 mg pyrantel embonate.
Precautions
SPECIAL WARNINGS FOR USE IN ANIMALS
For external use only.
Do not use on cats weighing less than 1 kg bodyweight.
Do not allow recently treated animals to groom each other.
Do not use in cases of hypersensitivity to the active substance.
There are no contraindications against use during pregnancy and lactation.
Care should be taken to avoid the contents of the tube coming into contact with the eyes or mouth of the recipient animal.
Occasionally a transient local reaction may be observed at the application site following treatment.
The product is bitter tasting and if the cat licks the site of application salivation may occur. This is not a sign of intoxication and will disappear within a few minutes of treatment.
Overdosing can lead to slight skin reactions which disappear without treatment within a few days.
If signs of disease persist or appear consult a veterinary surgeon.
USER SAFETY
The product can be an irritant to the skin and eyes.
Care should be taken to avoid the contents of the tube coming into contact with the skin or eyes.
If accidental contact with the skin or eyes occurs, wash off any skin contamination with soap and water immediately. Rinse the affected eyes thoroughly with clean, fresh water.
In the event of skin or eye contact, seek medical advice if irritation persists and show the Doctor this package.
Do not stroke or groom animals until the area of application is dry (typically around 1 hour after application).
Wash hands thoroughly after use.
Do not eat, drink or smoke during application.
Keep product in the outer carton.
Store away from food, drink and animal food.
Keep recently treated pets away from varnished, polished, plastic or leather surfaces.
KEEP OUT OF THE SIGHT AND REACH OF CHILDREN
Do not remove tablets from strip packing until required for use.
Any remaining half tablets should be discarded.
Do not use this veterinary medicinal product after the expiry date which is stated on the blister and carton after EXP.
Do not store above 25°C.
Keep foil blister in outer carton.
Disposal: Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal products should be disposed of in accordance with local requirements.






 Quick view
Quick view









